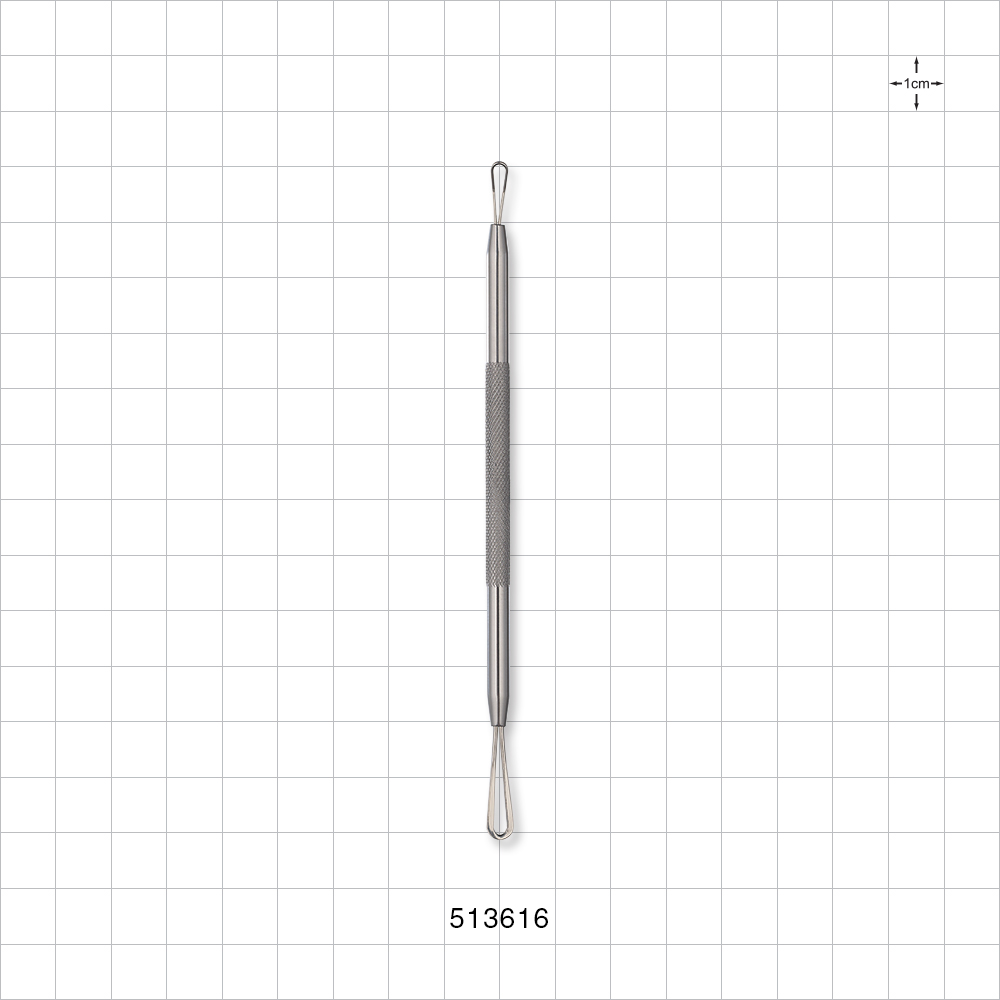
| Technical Detail | 4.8 inch (121.9 mm) |
| Materials | SS SS |
| Packaging | Individually Wrapped |
| Manufacturing Environment Classification | Controlled but not formally classified |
| Manufacturing Aids - Mold Release - Lubricant used | No |
| Colorant in Part | No |
| Regrind Used to Manufacture This Part | Information Not Available |
| Hazardous Goods | No |
| Product Shelf Life Non-Irradiation (months) | No shelf life indicated by the supplier |
| Animal Derived Ingredients (BSE/TSE) | Information Not Available |
| California Prop. 65 | Information Not Available |
| Conflict Minerals | Information Not Available |
| European Pharmacopoeia (EP) 3.1.X | Information Not Available |
| Human Derived Ingredients | Information Not Available |
| Latex | Does NOT intentionally contain natural latex |
| Latex in Packaging | Does NOT intentionally contain natural latex |
| Medicinal Substances | Information Not Available |
| Nanomaterials | Does NOT intentionally contain nanomaterials |
| Pyrogens | Not Tested for Pyrogens |
| RoHS3 (2015/863) | Information Not Available |
| EtO Compatible | Information Not Available |
| Gamma Compatible | Information Not Available |
| Steam Compatible | Information Not Available |
| X-Ray Compatible | Information Not Available |
| Biocompatibility tested ISO 10993 | No |
| USP Biocompatibility 87 | Information Not Available |
| USP Biocompatibility 88 | Information Not Available |
| Bioburden USP 61 62 | No bioburden information available |
| Tested via USP 661.1 | No |
The products in our inventory are intended solely for use as components for further manufacturing, processing or incorporation into a finished device. These products are not intended for use as finished devices. Customers are solely responsible for determining whether a Qosina component product is appropriate for customer’s intended use and for ensuring that the use of the component product is in compliance with applicable laws. Our components are not available for sale to hospitals, physicians, and pharmacists.