ISO 80369 Small-Bore Connector Standards
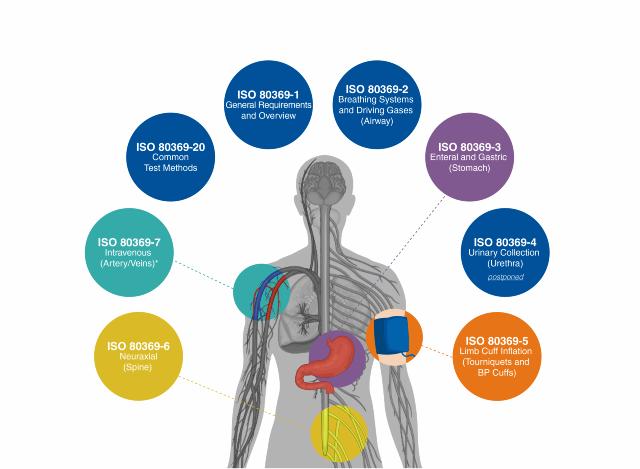
ISO 80369 is series of standards that were developed by the International Organization for Standardization to improve patient safety and reduce the risk of small-bore misconnections used in liquid and gas healthcare applications. Small-bore connectors convey liquids or gases in a patient-care setting, and are used in enteral, respiratory, urinary, blood pressure, neuraxial and intravenous systems.
Patients often have multiple lines connected to them to deliver medicine and nutrients. Numerous devices and systems use the universal luer lock connector, which is a type of small-bore connector. A small-bore connector is defined as having an ID of less than 8.5 mm, and a luer is defined by the ISO 594-1 and ISO 594-2 standards as “a conical fitting with a 6% taper for syringes, needles and certain other medical equipment.”
Through this connector, a healthcare worker or patient could mistakenly connect one device used for one application, such as enteral nutrition, to another device used for another application, such as intravenous therapy.
ISO 80369-1
General Requirements and Overview
ISO 80369-20
Common Test Methods
ISO 80369-2
Breathing Systems and Driving Gases (Airway)
ISO 80369-4
Urinary Collection (Urethra) - Postponed
ISO 80369-5
Limb Cuff Inflation (Tourniquets and BP Cuffs)
ISO 80369-7
Intravenous (Artery/Veins)*
*Note: Section 7 will define the standard luer fitting. This section will replace the current ISO 594-1 and the ISO 594-2. No physical changes to the current luer specification are anticipated.
How does this affect you?
If you are using any small-bore connectors as components in a kit, set or line that drives gas or pressurized fluids to patients and are using luers in non IV applications, this may impact you. ISO 80369 will involve some key changes from component suppliers, which may include designing new connectors or modifying existing connectors to meet the specifications in the standard.
ISO 80369-1 General Requirements and Overview
ISO 80369-1 specifies general requirements for small-bore connectors, which convey liquids or gases in healthcare applications. These small-bore connectors are used in medical devices or accessories intended for use with a patient. It also specifies the healthcare fields in which these small-bore connectors are intended to be used, including, but not limited to, applications for breathing systems and driving gases, enteral and gastric, urethral and urinary, limb cuff inflation, neuraxial devices, and intravascular or hypodermic.
ISO 80369-2 Respiratory
Changes Affect Two Designs for Respiratory Connectors
Respiratory connectors involve systems designed to administer breathing systems and driving gas applications. ISO 80369-2 connectors have designs for two small-bore connectors for respiratory applications. One is for use on low-flow equipment such as sample ports on anesthetic and ventilator breathing systems, and another is for use on high-flow equipment such as flowmeters, nebulizer therapy face masks and nasal cannulae.
ISO 80369-3 Enteral Feeding
Enteral is the first clinical application to make the transition to new, safer connectors. ISO 80369-3 enteral feeding connectors have been designed to improve patient safety and reduce the risk of feeding misconnections. The new standard has changed the configuration of the male and female connectors. The enteral connectors are larger and will not allow connectivity with the male luer or female luer connectors that meet the ISO 594 standards or any other connectors for any other clinical use. They also provide a locking feature that signals the appropriate connection and stays in place. Administration sets and syringes have a female connector end that fit into a male patient-access feeding tube port.
All enteral connectors including feeding tubes, administration sets, and medication, flush and bolus feed syringes are expected to comply with ISO 80369-3. Enteral-specific syringes will also be required to connect to the enteral feeding connector for medication, flush and bolus feed syringes.
What does Qosina offer to help you comply with ISO 80369-3
Qosina offers an extensive line of enteral feeding connectors that have been designed to meet the ISO 80369-3 standard. Qosina is offering ENFitTM male and female connectors, caps, stepped adapters, Y connectors, stopcocks, and syringes, as well as the ENFitTM to ENLock adapters, ENPlus cross spikes and universal bottle adapters for the ENPlus cross spike in various sizes and materials to meet the needs of your enteral project. Please visit our ENFitTM section at: www.qosina/ENFit
ISO 80369-4 Urinary and Urethral - Postponed
Future Changes for Urinary Catheters
Urethral applications involve the urinary catheters that are used to drain the bladder. This standard has been postponed.
ISO 80369-5 Limb Cuff
Development process ahead for limb cuff connectors
Limb cuff inflation connectors will join air inflation administration to limb cuffs.
ISO 80369-6 Neuraxial
This standard specifies requirements for small-bore connectors intended to be used in neuraxial applications. These applications involve the use of medical devices used to administer medications to neuraxial sites, wound infiltration anesthesia delivery and other regional anesthesia procedures, or to monitor or remove cerebro-spinal fluid for therapeutic or diagnostic purposes.
What does Qosina offer to help you comply with ISO 80369-6?
Qosina stocks a line of NRFit™ connectors and caps that are small and lightweight; made of medical-grade acrylic resin; BPA- and latex-free; gamma and EtO sterilization ready; and clear with no colorants added. The connectors are available to fit 0.083 inch (2.1 mm) or 0.122 inch (3.1 mm) outer diameter tubing, and the caps are offered in both vented and non-vented designs. The ergonomic wings on the female connectors and the easy-to-grip hub on the male connectors allow for ease of use. Please visit our NRFit™ selection at www.qosina.com/neuraxial.
ISO 80369-7 Intravascular or Hypodermic
ISO 80369-7 specifies dimensions and requirements for the design and functional performance of small-bore connectors intended to be used in intravascular or hypodermic applications of medical devices and accessories.
The ISO 80369-7 standard replaces ISO 594-1 and ISO 594-2, but it has been consolidated and technically revised. It is expected that all existing ISO 594 connectors will meet the requirements of ISO 80369-7 by 2021, and that when testing or validation occurs, it is made using the requirements of ISO 80369-7.
What does Qosina offer to help you comply with ISO 80369-7?
Qosina stocks a number of ISO 80369-7 compliant components including connectors, stopcocks, hemostasis valves and check valves. We are closely monitoring updates to this standard and adding new products on a regular basis. Please view our line of ISO 80369-7 components.
If you would like us to conduct any ISO 80369-7 testing on your behalf, or if you find a Qosina part that you like and it is not ISO 80369-7 compliant, please contact us to find out if we are able to test and/or modify the component. Additional fees may be required based on volume.
ISO 80369-20 Common Test Methods
ISO 80369-20 specifies the test methods to evaluate the performance requirements for small-bore connectors detailed in the ISO 80369 series. During the development of the ISO 80369 series of standards, it became evident that many of the test methods were very similar for each of the applications. It was therefore decided to standardize all test methods into a separate part of the series to prevent unnecessary duplication and minor differences. It is also recognized that not all connectors can be evaluated using each test method in this part. The test methods applicable to each connector are specified in the respective part of the ISO 80369 series.
How can Qosina help you prepare for the new standards?
Qosina actively follows ISO 80369 updates and adds compliant components to its extensive line as the standards evolve.
Where can I find additional and updated information?
International Organization for Standardization: www.iso.org
Association for the Advancement of Medical Instrumentation (AAMI): www.aami.org
For additional information, call a Qosina Customer Specialist at 631-242-3000 or email us at info@qosina.com
Note: This information is solely provided by Qosina based on research conducted through the ISO and AAMI websites. These notes have not been endorsed or approved by ISO or AAMI and should not be used to make specific business decisions.

