
Will USP <800> have an impact on medical device engineering?

When treating patients, hospital and healthcare personnel routinely encounter hazardous medications. The National Institute for Occupational Safety and Health (NIOSH) defines a drug as hazardous if it exhibits one or more of the following characteristics in humans or animals: carcinogenicity, teratogenicity or developmental toxicity, reproductive toxicity, organ toxicity at low doses, genotoxicity or structure and toxicity profiles of new drugs that mimic existing hazardous drugs2,3,4. For more information on drugs that are considered hazardous, see the NIOSH website1.
Traditionally, the hazards around drug management have been limited to compound pharmacy. U.S. Pharmacopeia general chapter USP <800> recognizes that there is a greater group of people that could have direct contact with these medications daily and need to be protected against the effects of these hazardous drugs1,4. This list not only includes the healthcare professionals who create and administer the drugs, but also delivery and cleaning personnel who may be at risk from damaged or contaminated containers.

Who is at risk?
- Pharmacists
- Pharmacy Technicians
- Nurses
- Physicians
- Surgeons
- Physician Assistants
- Respiratory Therapists
- Home Health Aides
- Nurses' Aides
- Housekeeping
- Janitorial Services
- Environmental Services
- Veterinarians
- Veterinarian Technicians
- Veterinarian Assistants
USP <800> was officially published as a general chapter on December 1, 2019. It outlines where exposure can occur and provides comprehensive guidance on various aspects of handling hazardous drugs, including receipt, storage, compounding, administration and disposal.
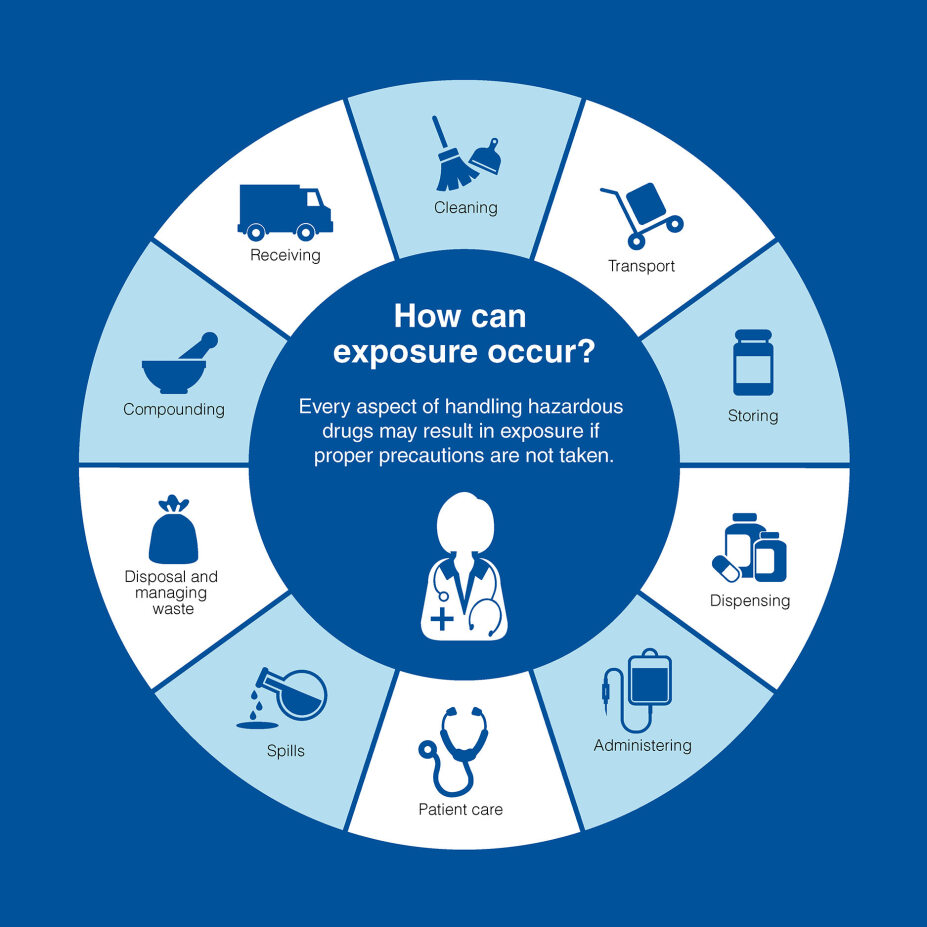
There are 11 key components to the chapter:
- Scope and purpose
- Definitions
- Risk assessment
- Facility and engineering controls
- Personal protective equipment (PPE)
- Compounding
- Administration and dispensing
- Waste management
- Training and competency
- Documentation
- Implementation
These components document how a risk analysis should be conducted at a facility to assess the possibility of drug exposure to the staff. The analysis should cover the full lifecycle of the hazardous drug, from receipt at the facility to administration and exiting the facility, either as a delivery to another facility or through waste disposal. Guidance is included on the type and amount of personal protective equipment that should be used at each stage by the facility's staff, how to create effective processes to document each stage and what should happen if a drug exposure or spillage occurs.
These are all supported by other standards within the U.S. Pharmacopoeia:
- USP <795> Pharmaceutical Compounding – Nonsterile Preparations
- SP SP <797> Pharmaceutical Compounding – Sterile Preparations
- USP <825> Radiopharmaceuticals – Preparation, Compounding, Dispensing and Repackaging
- USP <917> Radioactivity When Handling Radiopharmaceuticals
- USP <1207> Sterile Product Packaging
- USP <Weighing on an Analytical Balance – Accurate Weighing Techniques
- USP General Chapters <1000> - <1999> - Dosage Forms, Compounding Procedures and Equipment
- USP General Chapters <1200> - <1299> - Documentation Practices, Quality Assurance and Risk Management
Since its publication in 2019, USP <800> has been an “informational” standard. However, with the recent updates of USP <795> and <797>, USP <800> will become a federally enforced standard in November 2023. There will be a six-month grace period for facilities and their staff to ensure the requirements are fulfilled to meet this standard.
How does USP <800> impact medical device original equipment manufacturers (OEM)?
As USP <800> impacts hospital environments, it may be difficult to predict how this will impact the design and execution of medical devices meant for hospital use. Although medical devices will not need to be USP <800> compliant directly, considerations in the design phase will aid hospital decision-makers in sourcing the equipment that helps them meet the standard. These facilities will be searching for solutions to risks that could result in spills, splashes or surface contamination based on their risk analyses. Dust created during the dispensing or administration of hazardous drugs in pill form can be inhaled or can contaminate an area. This is just as hazardous as spillages of hazardous medications in liquid form.
Qosina is already starting to see instances where the outcome of these risk analyses has influenced product design.
A company that makes pill-crushing devices was asked to design a bag in which the pill could be sealed prior to crushing so that the nurses would not inhale dust particles from the pills. An oral or enteral syringe filled with fluid is attached to the bag in order to create a solution out of the pulverized medication. The attached syringe would then be used to extract the solution for patient administration. The simple yet clever addition of a luer to the bag to attach the syringe ensures the nurse is never exposed to the medication's particles or solution.
Additionally, there may be an increase in requests for infusion lines to become closed systems. Where hospital personnel were once willing to disconnect and reconnect infusion lines to flush the line or administer additional medication, the use of stopcocks or manifolds may now be requested in the design. Stopcocks or manifolds would reduce the need to disconnect the medication, which will, in turn, reduce the risk of exposure.
To meet USP <800>, any medical device that is used in the administration of a hazardous drug must be a closed system. The drug must be administered and the medical device disposed safely without any chance of contamination or exposure. This means that there should be no drug in dead spaces or tubing that could leak out. Apart from the addition of stopcocks, luer-activated valves, balloons, filters and other containment methods could be used to ensure that a medical device can be redesigned as a closed system and therefore ensure the safety of healthcare workers.
Developing a product that considers USP <800> not only ensures the safety and well-being of its users but also creates a unique selling point that addresses the facilities’ compliance requirements.
Contact Qosina to explore how you can develop a product that meets your customers’ requirements.
References
*Information and info graphics from the USP.org website USP 800 | USP
- https://www.cdc.gov/niosh/topics/hazdrug ↩
- Hansen J, Olsen JH. Scand J Work Environ Health. 1994 Feb;20(1):22-6 ↩
- Connor TH, et al. J Occup Environ Med. 2010 Oct;52(10):1019-27 ↩
- USP General Chapter Hazardous Drugs – Handling in Healthcare Settings. ↩
https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare

